
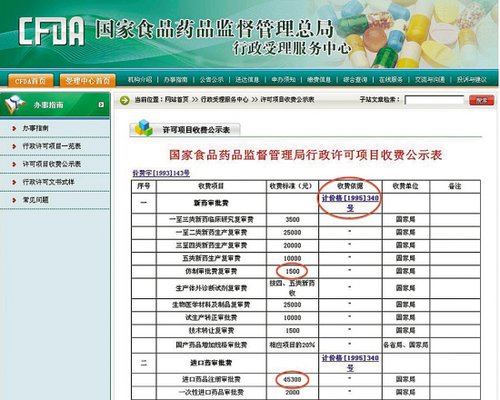
"Register pharmacy of a copy in China likewise, chinese native land pharmaceutical factory needs a RMB only 1500 yuan, and entrance medicine needs 45300 yuan. Exorbitant medicines and chemical reagents is registered cost and ' inequitable ' register a condition to make high grade international copy pharmacy is tasted, if come from the medicines and chemical reagents of the country such as the United States, Canada, India,enter Chinese market not easily. Such result makes Chinese dweller cannot enjoy cheap and high grade United States medicine, India medicine. Such result makes Chinese dweller cannot enjoy cheap and high grade United States medicine, India medicine..
Recently, have professing is " American small pharmaceutical factory " the netizen expects to newspaper of Yang Cheng evening paper, think exorbitant medicines and chemical reagents registers cost to make the international copy pharmacy of cheap of qualitative actor price cannot enter Chinese market, cause heat to discuss. Reporter of ovine wall evening paper investigates discovery, this is registered to expend for inequitable medicines and chemical reagents by bad-mouth, already did not change 18 years.
● current situation
Medicines and chemical reagents registers cost 18 years to did not change
Reporter of ovine wall evening paper supervises management board administration to permit a project to collect fees in national provision medicines and chemical reagents fair show a watch to go up (see right next graphs) see, new drug examines and approve cent to become 6 kinds, among them the 6th kind of copy pharmacy that often says namely. The ground is common show a list, the copy pharmacy of domestic company examines and approve cost to be 1500 yuan. And entrance mthe best spring pooledicines and chemical reagents, it is new drug or copy pharmacy witfree modern dining chairshout giving thought to, entrance medicines and chemical reagents is registered examine and approve cost to unite it is 45300 yuan.
But the reporter also arrives alertly at the same time, the basis that afore-mentioned collecting fees is state committee of planning, Ministry of finance is in what allotted on April 6, 1995 " examine and approve about adjusting medicines and chemical reagents, the announcement that check cheharrison hot spring dealcks and accept cost standard " (valuation case [1995]340 date) , this announcement is carried out since May 1, 1995, that is to say, this still is the rate 18 years ago. spring pool
What concept be 18 years? 18 years, national hair changes appoint (predecessor is a state committee of planning) the drug price that already started mornorth face shirtse than 30 rounds is adjusted. 18 years, of Chinese employee mean monthly income rises to make an appointment with 4000 yuan from about 400 yuan, can say, in those days 10 thousand yuan are equivalent to 100 thousand yuan of nowadays almost.
Since such, does medicines and chemical reagents register cost didn't rate go 18 years to whether had lagged behind? Should change after all?
● sound
1
Change! Look forward to of medicine of inside and outside wants fairness
The basis professes on the net is " American small pharmaceutical factory " the netizen's view, its are opposite the charge that the country feeds medicine to superintend total bureau to register medicines and chemical reagents in China to foreign pharmaceutical factory and requirement outclass the requirement of Chinese indigenous industry. Be like, it is to be in China to register pharmacy of a copy together, chinese native land pharmaceutical factory needs a RMB only 1500 yuan, and the United States and foreign pharmaceutical factory need 45300 yuan, foreign medicine look forward to is 30 times of medical look forward to more native land. Be like again, it is to register copy pharmacy together, indigenous industnorton internet antivirusry of China presses newspaper of pharmacy of the 6th kind of copy to approve, the regulation accords with after examine and verify, can send medicines and chemical reagents to approve article order directly. And whether is entrance medicines and chemical reagents copy pharmacy without giving thought to, the regulation accords with after examine and verify, send " clinical trial approves medicaments " , after clinical trial ends, make again examine and approve a decision.
To this, personage of look forward to of the traditional Chinese medicine outside having thinks these disobeyed administration to permit the provision with law fair fairness. Such rate needs a change, the principle that should follow openbrass faucet aerator , fair, justice will collect fees.
2
watch caseChange! Collect fees too low
Another kind of sound also thinks, active copy pharmacy examines and approve rate to want to change, the reason is, the rate 18 years ago, to now, collect fees too low.
A professional advisory corporation of Beijing that is engaged in medicines and chemical reagents be being registered domestic and internationally tells Yang Cheng evening paper reporter about the personage, bmodern dining chairsasis " medicines and chemical reagents registers administrative measure " , the development of look forward to of the medicine outside the condition that the country feeds medicine to superintend total bureau to be able to be approved to the newspaper and manufacturing situation undertake the spot is checked, and sample. "Although do not have regulation of proclaimed in writing, but the cost that actually these spot examinations require also is included in examine and approve cost in. That is to say, 45300 yuan when place of look forward to of foreign capital medicine hands in inside, included the charge that examines and approve staff official business to expedite, it is the country feeds medicine to superintend total bureau to do not have exercise all the time only such influence.
The personage inside course of study expresses, this one outside the clique is examined and approve carry out rarely really. Till 2011, medicines and chemical reagents registers ability to begin inspection of site of the production outside importing condition of medicines and chemical reagents first. The bureau of national medicine inspect at that time forms 7 examinations group, go to 7 countries such as the United States, France, Italy, India, Hungarian, Korea, Japan, to Bei Fazhu sheet fights fluid of inject of element of front row of Nuo of inject fluid, ground, inject to use hydrochloric acid auspicious 7 breed such as sitar bank begin the spot outside the condition to check the work, outside realizing condition of entrance medicines and chemical reagents, check " 0 " breakthrough.
"In fact, check requires cost to come from at public expense to site of the production outside the condition in those days, because with present price level, chan Ping examines and approve cost 45300 yuan of that collection from the enterprise, the inspection can't be produced outside supportive condition at all. " afore-mentioned personages say frankly, "The rate 18 years ago is returned after all nowadays comfortable do not suit, this is judged by medical inspect branch, the rate that just feels active inside course of study is not high. The rate that just feels active inside course of study is not high..
3
Change! Examine and approve too slow
"The charge threshold that medicines and chemical reagents registers is not high, medicines and chemical reagents examines and approve the biggest bottleneck is not to collect fees, however the qualified personnel of evaluation of medicines and chemical reagents that medical inspect branch provides is not worth badly, so that import drug,examine and approve speed too slow. Contrary, if rise,examine and approve cost to be able to be helped introduce a talent, accelerate examine and approve a process, carry efficient sentence, believe look forward to of medicine of a lot of foreign capital is willing to rise. " this is the sound that comes from look forward to of foreign capital medicine, also be the 3rd kind of sound.
Study a company according to Ai Meishi market (IMS) a research shows, the enrollment in medicines and chemical reagents level, wait for a reason as a result of manpower resource and flow limitation, chinese new drug appears on the market to compare other country late 4-5 on average or so years. IMS Chinavancouver to harrison hot springs area seeks advice from the chief Zhang Meng of business to express to the reporter, be in China, the enrollment of innovation medicine is approved, from take clinical admittance card to need the time of half one year probably.
Development of drug of association of company of investment of Chinese foreign trader and development industry committee carry out president Zhuo Yongqing to also express to the reporter, compare with international, american NDA (new drug applies for) provided 3000 much people, and the country feeds medicine to superintend totawatch case manufacturerl bureau careful to judge central chief editor to make only 120 people, among them personnel of examine and verify makes an appointment with 80 people. And those who form bright contrast is, feed medicine to superintend total bureau to release according to the country " medicines and chemical reagents is registered examine and approve year to report " , the medicines and chemical reagents 2009 registers application to accept gross to be 6428, accepted gross to add up to 2010 6294, accepted medicines and chemical reagents 2011 new register application 3620.
"This bit of person should handle 6000 many careful one year to judge, work overtime, it is very painful that these carefuls judge an expert. " Zhuo Yongqing also appeals, "Before 10 years are being mixed before current society follows 5 years, 20 have very big change before New Year, it is very special to the demand of innovation medicaments big, when national industry policy is changed, the constituent framthe most popular watch casesework system that is our government also should have a few changes. The constituent framework system that is our government also should have a few changes..
No comments:
Post a Comment